Purdue Pharma has another “killer” surprise for America — addicting 7 to 16 year olds!
 There is little outrage from the public in Purdue Pharma targeting children as young as 7 years old with yet another Holocaust of death in an FDA approved opioid.
There is little outrage from the public in Purdue Pharma targeting children as young as 7 years old with yet another Holocaust of death in an FDA approved opioid.
Marianne Skolek-Perez Global News Centre
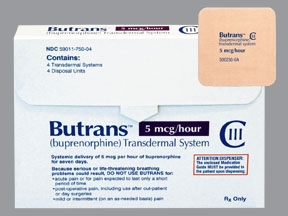
(MYRTLE BEACH) On September 14, the FDA Advisory Committee will be holding a hearing on Purdue Pharma’s new “blockbuster” opioid called Butrans. It is targeted for severe pain in children ages 7 to 16 years old. There is not a doubt in my mind that once the FDA approves this latest killer opioid presented by Purdue Pharma, it will be off-labeled marketed as OxyContin was for all pain levels. This time though children are the guinea pigs and Purdue Pharma and its owners, the Sacklers will in all probability double their profits.
Below is the FDA Advisory Committee posting on the hearing scheduled for September 14. Since the FDA functions completely with impunity — no accountability, conscience or responsibility to the safety of the American people, the FDA does not have to list who Purdue Pharma’s expert witnesses will be at the hearing. The hearing will also not be webcast to the public.
There is little outrage from the public in Purdue Pharma targeting children as young as 7 years old with yet another Holocaust of death in an FDA approved opioid. The organizations pushing for the use of Suboxone and Naloxone in the treatment of drug addiction in adults may want to pitch a dosage conversion table for these “miracle treatments” when children become hopelessly addicted to Butrans.
As a bit of history for those not knowledgeable, in the late 1990’s, a little known pharmaceutical company headquartered in Stamford, Connecticut unleashed a powerful prescription opioid called OxyContin — after approval by the FDA. OxyContin is infamous now as being a dangerous opioid responsible for tens of thousands of deaths and addictions throughout every state in the country. We now have states of emergency in the prescription opioid/heroin epidemic. The surge of heroin use has also been attributed to the widely prescribed OxyContin. Purdue Pharma’s revenues rose sharply over the years from approximately a few million dollars a year to billions of dollars a year thereby making the Sackler Family (owners of Purdue Pharma) one of the ten richest families in America.
In 2002 after losing my daughter to prescribed OxyContin, I made it my mission to learn everything I could about the marketing of OxyContin. My research led to working with the Department of Justice and charges being lodged against Purdue Pharma for the criminal marketing of OxyContin to physicians and patients as non-addictive. I also testified against the criminal pharmaceutical company in 2007 when they pleaded guilty in federal court to charges against them and also in front of the U.S. Senate Judiciary Committee.
As I was researching and uncovering more and more criminal marketing of OxyContin, I also was emailing and faxing correspondence to the FDA. The FDA over a short period of years had mailed Purdue Pharma no less than 12 warning letters about their less than honest marketing of OxyContin. At one point, I received a telephone call from Tom Abrams, director of the Office of Prescription Drug Promotion (OPDP). Mr. Abrams sounded as though he were annoyed that I would be questioning the lack of action by the FDA against Purdue Pharma. I challenged him with “how many warning letters do you plan on sending Purdue Pharma because Mr. Abrams, they don’t appear to be listening to you?” His reply stunned me. It was “We don’t have the manpower to police Purdue Pharma.” My closing remark to him was “And you don’t think Purdue Pharma doesn’t know that?” Mr. Abrams hung up on me.
How many warning letters do you imagine the FDA will be sending Purdue Pharma about the criminal marketing of Butrans? A blind eye by the public compliments of Purdue Pharma and the FDA — a perfect storm for your children’s worst nightmare. Scary? You bet it is.
UPDATED PUBLIC PARTICIPATION INFORMATION: September 14, 2017: Joint Meeting of the Anesthetic and Analgesic Drug Products Advisory Committee and the Drug Safety and Risk Management Advisory Committee Meeting Announcement
| Center | Date | Time | Location |
|---|---|---|---|
| CDER | September 14, 2017 | 8:00 a.m. to 12:30 p.m. |
Tommy Douglas Conference Center 10000 New Hampshire Avenue Silver Spring, Maryland 20903 |
Agenda
Meeting Materials
Public Participation Information
- Federal eRulemaking Portal: https://www.regulations.gov. Follow the instructions for submitting comments. Comments submitted electronically, including attachments, to https://www.regulations.gov will be posted to the docket unchanged. Because your comment will be made public, you are solely responsible for ensuring that your comment does not include any confidential information that you or a third party may not wish to be posted, such as medical information, your or anyone else’s Social Security number, or confidential business information, such as a manufacturing process. Please note that if you include your name, contact information, or other information that identifies you in the body of your comments, that information will be posted on https://www.regulations.gov.
- If you want to submit a comment with confidential information that you do not wish to be made available to the public, submit the comment as a written/paper submission and in the manner detailed (see “Written/Paper Submissions” and “Instructions”).
- Mail/Hand delivery/Courier (for written/paper submissions): Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852.
- For written/paper comments submitted to the Division of Dockets Management, FDA will post your comment, as well as any attachments, except for information submitted, marked and identified, as confidential, if submitted as detailed in “Instructions.”
- Confidential Submissions-To submit a comment with confidential information that you do not wish to be made publicly available, submit your comments only as a written/paper submission. You should submit two copies total. One copy will include the information you claim to be confidential with a heading or cover note that states “THIS DOCUMENT CONTAINS CONFIDENTIAL INFORMATION.” The Agency will review this copy, including the claimed confidential information, in its consideration of comments. The second copy, which will have the claimed confidential information redacted/blacked out, will be available for public viewing and posted on https://www.regulations.gov. Submit both copies to the Division of Dockets Management. If you do not wish your name and contact information to be made publicly available, you can provide this information on the cover sheet and not in the body of your comments and you must identify this information as “confidential.” Any information marked as “confidential” will not be disclosed except in accordance with 21 CFR 10.20 and other applicable disclosure law. For more information about FDA’s posting of comments to public dockets, see 80 FR 56469, September 18, 2015, or access the information at: http://www.fda.gov/regulatoryinformation/dockets/default.htm.
Webcast Information
Contact Information
- Stephanie L. Begansky, PharmD
Center for Drug Evaluation and Research
Food and Drug Administration
10903 New Hampshire Avenue
WO31-2417
Silver Spring, MD 20993-0002Phone: 301-796-9001
Fax: 301-847-8533
E-mail: [email protected]
- FDA Advisory Committee Information Line
1-800-741-8138
(301-443-0572 in the Washington DC area)- follow the prompts to the desired center or product area
Please call the Information Line for up-to-date information on this meeting.
__________________________________________________________________________________________
 Global News Centre’s Marianne Skolek, is an Investigative Reporter who focuses on the Prescription Opioid/Heroin Epidemic in the U.S. and Canada. In particular, Marianne has covered the criminal marketing of OxyContin going back to 1999 and continuing to the present.
Global News Centre’s Marianne Skolek, is an Investigative Reporter who focuses on the Prescription Opioid/Heroin Epidemic in the U.S. and Canada. In particular, Marianne has covered the criminal marketing of OxyContin going back to 1999 and continuing to the present.
In 2002, Marianne lost her daughter, Jill to prescribed OxyContin which her physician referred to as “mobility in a bottle.” It was, in fact, death in a bottle. After doing extensive research on the maker of OxyContin, Purdue Pharma, Marianne began working with the Department of Justice in Virginia in their criminal investigation into Purdue Pharma and in July 2007 was asked by the U.S. Attorney John Brownlee prosecuting the case to testify against the three CEO’s of Purdue Pharma, Michael Friedman, Paul Goldenheim, MD and Howard Udell, Chief Counsel. The CEO’s pleaded guilty to misleading the medical profession about the dangers of OxyContin. Marianne also testified against Purdue Pharma at a Judiciary Hearing of the U.S. Senate in July 2007.
In addition, a dangerous and highly addictive opioid named Zohydro has been approved by the FDA against their Advisory Committee’s advice and Marianne continues to alert Attorneys General, Senators and Congressmen as to the FDA’s irresponsibility in the out of control prescription opioid/heroin epidemic killing and addicting in the tens of thousands each year. Zohydro has been referred to as “heroin in a capsule” and its lowest dosage (10mg) contains twice as much hydrocodone as found in a Vicodin pill. The highest single dose of Zohydro contains as much hydrocodone as 5 to 10 tablets of Vicodin or Lortab. Zohydro mixed with alcohol can be fatal and has no abuse deterrent built in which will make it easy to crush and deliver a fatal dose of the opioid.
Currently Marianne has been instrumental in calling for the termination of Margaret Hamburg, MD, Commissioner of the FDA as well as Bob A. Rappaport, MD and Douglas Throckmorton, MD for their lack of commitment to safeguarding the American public against the prescription opioid/heroin epidemic. Marianne’s research, writing and contact with government agencies and attorneys has also exposed the heavily funded pain foundations set up by the pharmaceutical industry and their paid physician spokespersons who convinced the medical boards in 50 states and Canada that dangerous opioids such as OxyContin were less likely to be addictive. These physicians — in particular Scott Fishman, MD, J. David Haddox, DDS, MD, Perry Fine, MD, Lynn R. Webster, MD, Russell Portenoy, MD also downplayed the risks of addictive opioids in books as authors. These books are still available for sale and promoted to the medical profession.
Here are links to Marianne’s involvement in exposing the national conspiracy of the prescription opioid/heroin epidemic, the FDA, the pharmaceutical industry, their pain foundations and paid physician spokespersons.
http://www.salem-news.com/by_author.php?reporter=Marianne%20Skolek
http://www.youtube.com/watch?v=tmPG1VjD61U&list=UUWoHUEr4ZAbQOfIqtOArjgg&index=6&feature=plcp
https://www.facebook.com/photo.php?v=855537227796352&set=vb.658186307531446&type=2&theater



































Leave a Reply
You must be logged in to post a comment.